MEDISAFE Project
Objective
-
€ 3,9mBUDGET
-
26/03/2018PROJECT START
-
36 monthsDURATION
Expertise France is leading a European consortium for the implementation of this regional project which aims to assist countries in establishing structural measures to strengthen the prevention, detection and response to falsified medicines in East and Central Africa. MEDISAFE also aims to strengthen regional cooperation on harmonising policies and legislation, and to improve coordination and joint operations to fight against the production and trafficking of falsified medicines.
Falsified medicines: a multifaceted global problem
The production, trafficking and consumption of falsified medicines pose a threat to public health and safety. Falsified medicines are fake medicines packaged as approved drugs. They may contain poor quality or incorrectly dosed ingredients, including active ingredients, with a quantity that is too high or too low. In some cases, they may contain ingredients that are toxic or harmful to health. Consequently, they may fail to treat, cause harm or even kill consumers. Falsified medicines are a major threat to global public health and safety. While no country is protected against this threat, there is a particularly strong impact in developing countries, where falsified medicines are found in the treatment of life-threatening diseases such as malaria, tuberculosis and HIV/AIDS.
The World Health Organization (WHO) estimates that falsified drugs account for between 10 and 15% of the pharmaceutical market worldwide. In certain regions in Africa, Asia and Latin America, falsified medicines can account for up to 60% of medicines in circulation. The appeal of high financial benefits, combined with the low risk of prosecution and relatively lenient sentences, make the production of falsified medicines an interesting business for criminal groups. This parallel industry today accounts for several billion dollars.
The fight against falsified pharmaceutical products comes up against several challenges:
• A lack of consensus on definitions,
• A lack of reliable and scalable technology to detect fake drugs before they reach patients,
• The weakness of regulatory authorities at the global and national levels in combating this scourge, and
• Shortcomings in regulations and controls on manufacturing, in particular in China and India, where falsified products often come from.
Significant progress has been made, particularly in terms of regulations, with, for example, the MEDICRIME Convention, signed in Moscow in 2011, which was drafted to protect vulnerable patients and their right to secure access to medicines of adequate quality, and fight against organised crime. Another example is the Zanzibar Declaration on falsified medicines and pharmaceutical crime in 2010, in which Burundi, Kenya, Rwanda, Tanzania, Uganda and Zanzibar pledged to strengthen the fight against drug falsification and other pharmaceutical crimes in East Africa.
A comprehensive and integrated approach
To address these issues, the European Union has set up MEDISAFE, a project to fight against falsified medicines in 11 East and Central African countries.
Expertise France has been selected by the European Commission to implement the project at the head of a consortium of 7 partners, including AIFA (Agenzia Italiana del Farmaco / Italian Medicines Agency), the Medicines and Development Network (REMED), the Humanitarian Centre of Pharmacy Professionals (CHMP), the International Conference of Francophone Pharmacists Associations (CIOPF), the Chirac Foundation, ASST Fatabenefratelli Sacco and the Professional Association of Public Health Pharmacist Inspectors (APRO-PHISP). The advisory board includes WHO, OCLAESP and the Dutch National Institute for Public Health and the Environment (RIVM).
The consortium’s strategy is to provide a holistic response, on the basis of WHO recommendations and capitalising on projects implemented in the region, in particular in the context of the CBRN Centres of Excellence, one of the objectives of which is to support the fight against the production and trafficking of falsified medicines and falsified pharmaceutical products.
|
|
The project objectives are based on 3 pillars which aim to strengthen prevention, detection and response in terms of falsified medicines:
• By preventing the manufacturing, sale and consumption of falsified medical products in order to guarantee the quality and safety of the medicines distributed;
• By setting up systems to detect products that are falsified or do not comply with standards in distribution channels;
• By providing responses to guarantee the protection of public health.
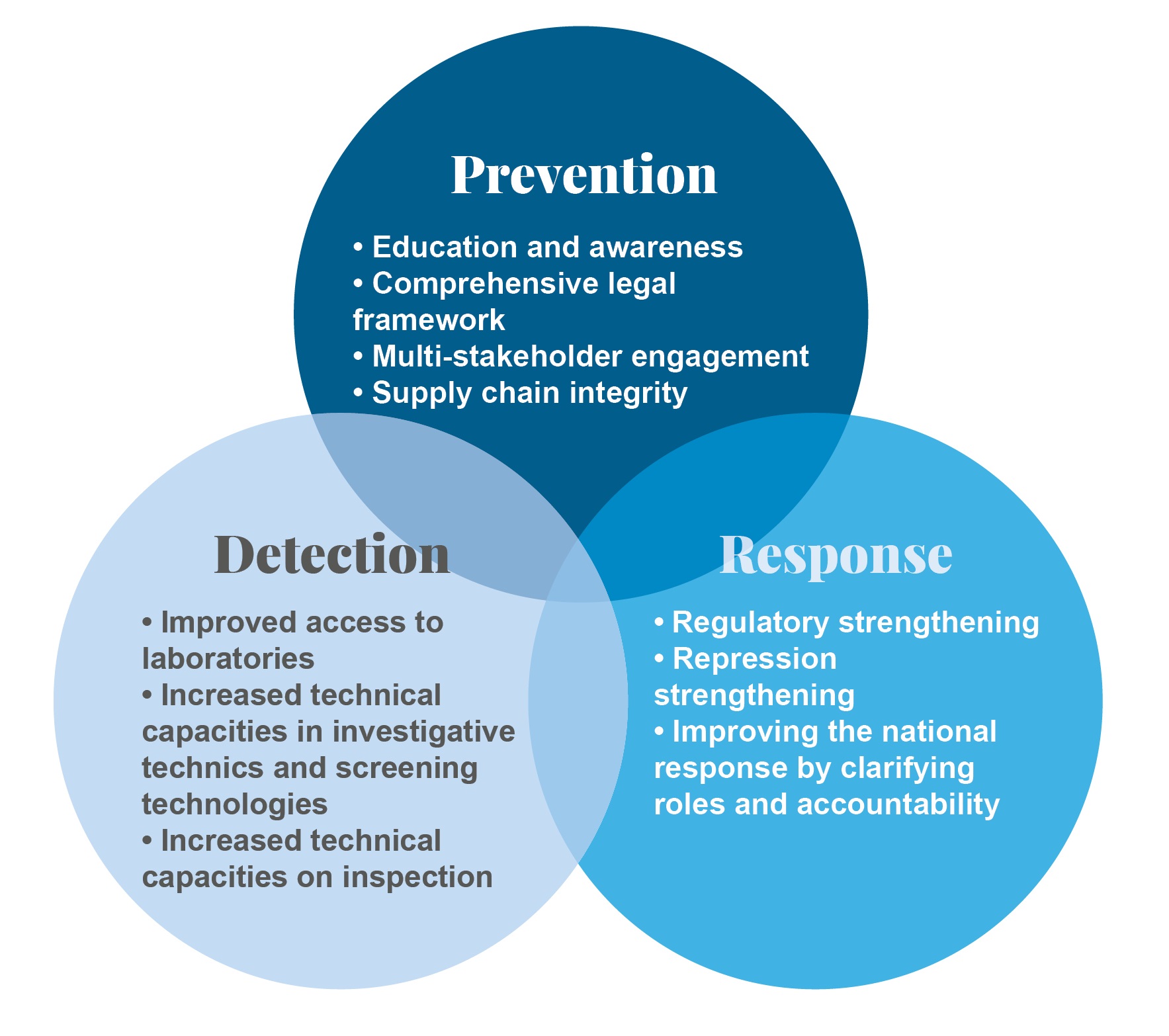
A strategy for action with several components
The actions implemented in the context of these three pillars will comprise:
• A preparatory phase with all the local and international partners, to collect data capitalised on the projects carried out, map the actors of CBRN and refine the action plan at the local and regional levels;
• Awareness-raising activities conducted with legal and medical actors and the general public via seminars and working and information sessions;
• A technical assistance phase for the development of national legal systems harmonised at regional level, for the prevention and effective repression of the manufacturing, transport and sale of falsified medicines;
• Capacity building for professionals from the health sector and pharmaceutical industry and legal and civil society actors for the detection and control of falsified medicines, via training and exchange workshops and the creation of a group of experts at regional level;
• Work on improving inter-agency cooperation (pharmaceutical industry, ministries, customs, police, etc.) and international cooperation (WHO, Interpol, etc.) in order to avoid the duplication of activities already conducted and to clearly establish the roles and responsibilities of each actor, in particular through the adoption of action plans at the national level and a regional platform for exchanges;
• Strengthening the security of the supply chain (physical and online) by mapping the issues and actors, and setting up mechanisms to detect and fight against falsified medicines.
The impact and sustainability of the project are ensured through the mobilisation of experts recognised in the fight against pharmaceutical crimes and in international cooperation, by involving regional focal points and drawing directly on tools and lessons learned from projects already conducted to fight against falsified medicines.
 To follow MEDISAFE news: www.medisafe-p66.eu
To follow MEDISAFE news: www.medisafe-p66.eu









 MEDISAFE is part of the
MEDISAFE is part of the 